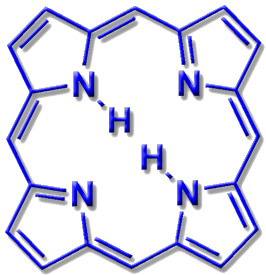
Porphyrin
The heterocycle protoporphyrin and its derivatives can be found in the active sites of many enzymes. The coordination of metal ions in the macrocyclic ring depends on the size of the open space in the ring and the ionic radius of the metal ion. Depending on the size of the ion, metal ions can be coordinated in the plane of the ring or slightly above it (in-plane or out-of-plane). The macrocycle is then doubly deprotonated.
Variations of the ring skeleton allow a certain amount of variation of the ring size in order to adapt to the requirements of the metal ions (cf. Corrin).
Schematic depiction |
Metal ions and their coordination to protoporphyrins |
||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||
|
|
Note: links will open in a new browser window. |