Siderophores
In inorganic nature, iron is generally found as Fe3+ ions in minerals. The metal is tightly bound in these materials, so that special ligand systems are needed in order to make this element, which is essential to nearly all organisms, biologically available.
Such ligands are called siderophores. They are able to form Fe3+ complexes with very high formation constants (up to 1052 for enterobactin). In this way, the practically insoluble "inorganic" iron can be removed from minerals and absorbed through very specific transport mechanisms.
The intracellular release of the (very tightly bound) iron takes place via pH or redox dependent reactions.
In siderophores, the Fe3+ ions can be bound to different functional groups. Typical ligand systems are hydroxamates, catecholates or a combination of carboxylate and amino groups of amino acids.
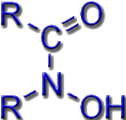
Hydroxamate Siderophores |
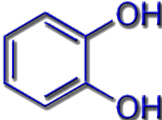
Catecholate Siderophores |
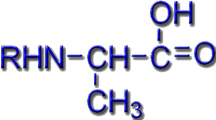
Amino Acid Siderophores |
|
|
|
|
|
e.g. in Coprogen and in Ferrichrome A |
e.g. in Enterobactin |
e.g. in Mugineinic acid |