Iron Uptake
Although iron is the fourth most common chemical element in the Earth's crust, its bioavailability is relatively low. In the oxygen atmosphere of Earth, practically all of the iron is found in the oxidation state +3. The solubility product for Fe(OH)3 is, at 2 • 1039 mol4 • l-4, extremely small, so that especially in soils with high pH (for example in regions where the stone is mostly limestone or dolomite), iron deficiency is common. The practically insoluble Fe3+ becomes available by "complex chemical mobilisation." Especially microorganisms are able to produce special ligands for Fe3+.
These ligands are referred to as siderophores and form octahedral Fe3+ complexes with extremely high (thermodynamic) stability. Remobilisation is achieved by taking advantage of the (kinetic) lability of such compounds and/or by redox processes, since most siderophores bind exclusively to Fe3+.
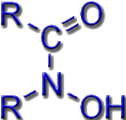
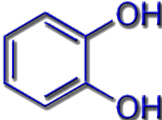
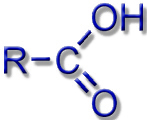
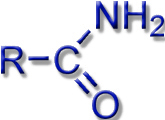
Most known siderophores are based on the same chemical building blocks (ligand systems): hydroxamates, catecholates, or protein building blocks.
Hydroxamates |
Catecholate |
Carboxylates, Amines, Amides |
|
|
|
|