Crown Ethers
Model Compounds for Size Selection of Singly Charged Cations
For transport in biological systems, alkali ions are bound by special ligands, called ionophores. These ligands show remarkable ion selectivity, which is usually determined by the size of the corresponding cations.
Similar selectivity can be observed by crown ether complexes, which selectively bind different alkali metal ions depending on the ring size.
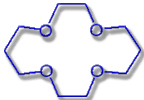
14-Crown-4 |
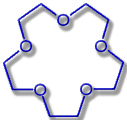
15-Crown-5 |
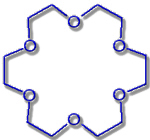
18-Crown-6 |
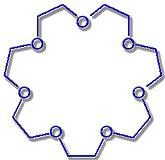
21-Crown-7 |
|
|
|
|
|
|
Hole diameter: 120-150 pm |
Hole diameter: 170-220 pm |
Hole diameter: 260-320 pm |
Hole diameter: 340-430 pm |
|
|
|||
Li+-Ion: Ø 152 pm |
Na+-Ion: Ø 204 pm |
K+-Ion: Ø 276 pm |
Rb+-Ion: Ø 304 pm |
Click on the pictures of the ions to see the structures of the complexes.